JUMP TO TOPIC
Spectator Ions Calculator + Online Solver With Free Steps
The Spectator Ions Calculator is used to identify the spectator ions in a chemical reaction. The spectator ions are the ions that do not take part in the chemical reaction.
They remain unchanged and are found in both the reactants and the products. The calculator takes the chemical equation as input and outputs the net ionic equation.
What Is a Spectator Ions Calculator?
The Spectator Ions Calculator is an online tool that is used to identify the spectator ions in an ionic equation of a chemical reaction.
The physical states of all the reactants and products are essential in identifying the spectator ions. (aq) represents the substance is soluble in water and (s), (l), and (g) represents the solid, liquid, and gaseous state respectively.
These states tell whether the ions have changed their states from reactants to products thus determining the spectator ions.
How To Use the Spectator Ions Calculator
The user can follow the steps given below to use the Spectator Ions Calculator.
Step 1
The user must first enter the chemical equation for which the spectator ions are required. It should be entered in the input block labeled, “Enter the Equation”.
Step 2
The user must then press “Submit” for the calculator to process the chemical equation.
Output
After processing the input, the calculator displays the output which consists of the following windows.
Input Interpretation
This window shows the input chemical equation that the calculator interpreted.
Balanced Equation
In this window, the input chemical equation is balanced and displayed. A balanced chemical equation is an equation with an equal number of elements on both sides of the reactants and products.
Structures
This window shows the chemical structures displaying all the chemical bonds present in all the substances of the chemical reaction. It also shows the charges on the ions of the ionic compounds.
Word Equation
This window shows the word equation for the input chemical reaction. The names of all the substances are present in the word equation.
Equilibrium Constant
The reactants and products define the equilibrium constant Kc from the balanced chemical equation. It can be written as follows:
\[ K_c = \frac{ {[P_{1}]}^{M_{P_{1}}} {[P_{2}]}^{M_{P_{2}}} }{ {[R_{1}]}^{M_{R_{1}}} {[R_{2}]}^{M_{R_{2}}} } \]
Where,
$M_{P_{1}}$ = No. of moles of the first product P1
$M_{P_{2}}$ = No. of moles of the second product P2
$M_{R_{1}}$ = No. of moles of the first reactant R1
$M_{R_{2}}$ = No. of moles of the second reactant R2
Rate of Reaction
The rate of reaction is the rate at which the chemical reaction takes place. It is also obtained from the balanced chemical equation.
The reactants and products are divided by $\Delta t$ along with the number of moles in the balanced equation.
The calculator computes the rate of reaction by assuming constant volume and no accumulation of intermediated and side products.
Chemical Names and Formulas
The calculator also displays the names, IUPAC names, chemical formulas, and Hill’s formula for all the compounds present in the chemical reaction.
Substance Properties
The calculator also computes various chemical and physical properties of all the substances in the chemical equation.
These properties include molar mass, phase, melting point, boiling point, density, solubility in water, and odor.
Solved Examples
Here are some examples solved through the Spectator Ions Calculator.
Example 1
For the chemical reaction:
\[ { Fe Cl_{3} }_{(aq)} \ + \ {Na OH}_{(aq)} \ \longrightarrow \ { Fe (OH)_{3} }_{(s)} \ + \ { NaCl }_{(aq)} \]
Find the net ionic equation and also identify the spectator ions in the chemical reaction.
Solution
The user must first enter the chemical equation as stated in example 1. The states can be removed while entering the chemical equation.
After submitting the input equation, the output window opens which firstly shows the input interpretation. The calculator shows the interpreted equation in this window as follows:
\[ Fe Cl_{3} \ + \ Na OH \ \longrightarrow \ Fe (OH)_{3} \ + \ NaCl \]
The next window shows the Balanced Equation from the input chemical equation as follows:
\[ Fe Cl_{3} \ + \ 3 Na OH \ \longrightarrow \ Fe (OH)_{3} \ + \ 3 NaCl \]
The calculator shows the chemical anatomy of all the substances present in the chemical equation.
The chemical structure for $Fe Cl_3$ is as follows:
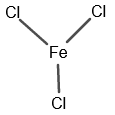
Figure 1
NaOH consists of one $Na^{+}$ and $OH^{-}$ ion.
$Fe (OH)_{3}$ is an ionic compound consisting of one $Fe^{+3}$ and three $OH^{-}$ ions
NaCl consists of one $Na^{+}$ and $Cl^{-}$ ion.
The spectator ions in this equation are three $Cl^{-}$ and three $Na^{+}$ ions.
The word equation for the chemical reaction is as follows:
\[ Iron (Ⅲ) \ Chloride \ + \ Sodium \ Hydroxide \ \longrightarrow \ Iron (Ⅲ) \ Hydroxide \ + \ Sodium \ Chloride \]
The Equilibrium Constant Kc is given as:
\[ K_c = \frac{ [ Fe (OH)_{3} ] \ [ NaCl ]^{3} }{ [ Fe Cl_{3} ] \ [ NaOH ]^{3} } \]
The calculator also computes the equation for the Rate of Reaction. It is given as follows:
\[ Rate = – \frac{ \Delta [ Fe Cl_{3} ] }{ \Delta t} = – \frac{1}{3} \frac{ \Delta [NaOH] }{ \Delta t} = \frac{ \Delta [Fe (OH)_{3}] }{ \Delta t} = \frac{1}{3} \frac{ \Delta [NaCl] }{ \Delta t} \]
The calculator also displays the chemical names and formulas as shown in Table 1:
| Iron (Ⅲ) Chloride | Sodium Hydroxide | Iron (Ⅲ) Hydroxide | Sodium Chloride | |
| Formula | $Fe Cl_{3}$ | NaOH | $Fe (OH)_{3}$ | NaCl |
| Hill Formula | $Cl_{3} Fe$ | HNaO | $H_{3} Fe O_{3}$ | Cl Na |
| Name | Iron (Ⅲ) Chloride | Sodium Hydroxide | Iron (Ⅲ) Hydroxide | Sodium Chloride |
| IUPAC Name | Trichloroiron | Sodium Hydroxide | Ferric Trihydroxide | Sodium Chloride |
Table 1
The calculator also displays the Substance Properties of all the reactants and products. These are shown in Table 2:
| $Fe Cl_{3}$ | NaOH | $Fe (OH)_{3}$ | NaCl | |
| Molar Mass g/mol | 162.2 | 39.997 | 106.87 | 58.44 |
| Phase (at STP) | solid | solid | solid | |
| Melting Point (°C) | 304 | 323 | 801 | |
| Boiling Point (°C) | 1390 | 1413 | ||
| Density ($g/cm^{3}$) | 2.13 | 2.16 | ||
| Solubility in Water | soluble | soluble | ||
| Surface Tension (N/m) | 0.07435 | |||
| Dynamic Viscosity (Pa.s) | 0.004 | |||
| Odor | odorless |
Table 2
All the images are created using Geogebra.
