JUMP TO TOPIC
Oxidation State Calculator + Online Solver With Free Steps
The Oxidation State Calculator is used to calculate the oxidation state or the oxidation number of a single element or all the elements in a compound. The oxidation state defines the number of electrons lost or gained by the element in the chemical process. The ionic compounds contain elements that lose or gain electrons to form ionic bonds.
The element which loses electrons in the chemical reaction has a positive oxidation number and the element that gains electrons is assigned a negative oxidation number.
The oxidation state of a pure element is always zero as it didn’t react to form an ion. The positive ion is referred to as the cation and the negative ion as the anion.
Early on, the term “Oxidation” was used only for the reaction of oxygen with different elements. It was observed that oxygen always gains electrons while reacting with electropositive elements. Later, the term oxidation was broadened and used for all the elements ionized in a chemical reaction.
The oxidation state defines the electropositive or electronegative nature of an element in a compound. The charge on each of the elements defines how many electrons are lost or gained by the element by the ion.
The oxidation state or number is the charge on the individual ionic element. For example, in the peroxide ion $ { O_{2} }^{-2} $, the oxidation state of each oxygen atom is -1. In the superoxide ion $ { O_{2} }^{-1} $, the oxidation number of the individual oxygen atom is -1/2.
In a monoatomic ion such as $ Na^{+1} $, the oxidation state is the same as the charge on the ion. In an ionic compound such as $Mg Cl_2$, the oxidation state of magnesium and chlorine should be such that the net charge on the compound is zero.
As chlorine is a highly electronegative element, it has an oxidation state to be -1. The two chlorine atoms make the oxidation number multiplied by 2 which becomes -2.
For the net charge to be zero, the alkaline earth metal magnesium should have the oxidation state to be +2. The calculator computes the oxidation states for all the elements in the compound.
What Is an Oxidation State Calculator?
The Oxidation State Calculator is an online tool that is used to compute the oxidation states of all the elements in the compound by entering the chemical formula for the particular ionic compound.
It also computes the oxidation numbers of all the elements in different ions.
For example, the sulphate ion has the formula as $ {SO_{4} }^{-2} $. The calculator will compute the oxidation states of sulfur and oxygen in the sulfate ion.
It also displays the atomic structure of the compound with the highlighted oxidation states.
How To Use the Oxidation State Calculator
The user can use the Oxidation State Calculator by following the steps given below.
Step 1
The user must first enter the chemical formula for the ion or compound whose oxidation states of the elements are required. It should be entered in the input block labeled as “Type formula here”.
Step 2
After entering the chemical formula for the ionic compound or the particular ion, the user must press the “Submit” button.
The calculator processes the input formula and computes the oxidation states of all the elements in the formula.
Output
The Oxidation State Calculator displays the output in the four windows given below.
Input Interpretation
The calculator interprets the input and displays the name of the compound whose chemical formula is entered by the user.
Result
The calculator computes the oxidation states of all the elements and displays them in this window. It also shows the structure of the molecule or compound as entered by the user.
The molecule contains the same atoms of an element whereas a compound has a variety of elements.
Chemical Names and Formulas
This window shows the formula of the ionic compound entered by the user. It also shows Hill’s formula, name, and IUPAC name of the entered chemical formula.
Ion Equivalents
The calculator also shows the ion equivalents if the user enters the formula for an ionic compound. The ion equivalents show the number of cations and anions present in the ionic compound.
It also shows the individual oxidation states of all the ions in the ionic compound.
Solved Examples
The following examples are solved through the Oxidation State Calculator.
Example 1
For the ionic compound magnesium hydroxide, calculate the oxidation states of magnesium, oxygen, and hydrogen in the compound.
Solution
The chemical formula for magnesium hydroxide is $ Mg (OH)_2 $. The user must first enter the chemical formula in the input window of the Oxidation State Calculator.
After submitting the input formula, the calculator computes the output in the following four windows.
The Input Interpretation window shows the name of the ionic compound as entered by the user. For this example, it shows “magnesium hydroxide”.
The calculator computes the oxidation states of magnesium, oxygen, and hydrogen to be +2, -2, and +1 respectively shown in the result window.
The molecular structure of magnesium hydroxide contains two hydroxide ions $ OH^{-1} $ having two ionic bonds with one magnesium ion $ Mg^{+2} $.
The calculator only shows the ions separately in this window.
Hill’s formula for magnesium hydroxide is $ H_2 Mg O_2 $ and the IUPAC name is magnesium dihydroxide.
The Ion Equivalents show that there is one magnesium cation and two hydroxide anions in magnesium hydroxide.
Example 2
Calculate the oxidation states of phosphorous and oxygen in the phosphate ion.
Solution
The chemical formula entered by the user for phosphate ion is $PO_{4}$.
After submitting the input formula, the calculator shows interprets the input and displays the name for the entered ion. It shows “phosphate anion”.
The calculator computes the oxidation state of phosphorous as +5 and that of oxygen as -2 as displayed in the result window.
The structure of phosphate ion is shown in figure 1.
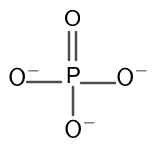
Figure 1
All the images are created using Geogebra.
