JUMP TO TOPIC
Lewis Structure Calculator + Online Solver With Free Steps
The Lewis Structure calculator is used to draw the Lewis dot structures of various molecules. It takes the molecule as input and outputs its Lewis dot structure.
The Lewis theory is a theory proposed by Lewis for covalent bonding and drawing structures of the various compounds. Many other theories were proposed after this theory for covalent bonding.
The further theories were the Valence Bond Theory VBT, Valence Shell Electron Pair Repulsion Theory named VSEPR Theory, and Molecular Orbital Theory abbreviated as MOT.
This calculator provides the structure of the molecule with all the covalent bonds present in accordance with Lewis’s theory.
It shows the valence electrons of each atom in a molecule according to the octet rule in the Lewis Structure model. The calculator shows the elaborate structure of the molecule entered in its input window.
What Is a Lewis Structure Calculator?
The Lewis Structure calculator is an online tool that is used to configure the atoms in a molecule for covalent bonding. It draws the structure of the molecule by the defined rules of Lewis’s theory of covalent bonding.
Covalent bonding is an important concept in the field of chemistry. It is studied by many chemists in history and many theories have been proposed. The first theory proposed for covalent bonding was the Lewis theory.
A covalent bond is defined as the sharing of electrons between atoms of a molecule. A single covalent bond results in the sharing of a single electron from both the atoms and double covalent bond results in the sharing of two electrons from both the atoms in a molecule and so on.
To understand the Lewis Structure Calculator, the user needs to understand the Lewis theory for covalent bonding. The Lewis structure is based on two principles.
The first principle of the Lewis model states that the structure is drawn only considering the electrons in the outer-most bond. The Lewis model represents the valence electrons of each atom by dots hence called the Lewis dot structure.
The second principle of Lewis’s Structure takes the fact that the valence shell of an atom can only accommodate eight electrons. This is known as the octet rule. Hydrogen has the exception of a maximum of two electrons in the valence shell.
The third principle of the Lewis Structure Theory indicates that the octet rule can be neglected for the central atom provided that the central atom can have eight or more electrons in its valence shell but not less than eight electrons.
The Lewis theory gives the basic concept for electron sharing between atoms of a molecule. It demonstrated the basic structure which many chemists studied further to invent new theories related to covalent bonding.
The user enters the molecule and the calculator draws the Lewis Dot Structure for the particular molecule. This tool is quite useful for chemistry students to draw the Lewis Dot Structure and understand the covalent bonds between atoms of the molecule.
How To Use Lewis Structure Calculator
You can use the Lewis Structure Calculator by following the steps given below to configure the Lewis Structure of any molecule.
Step 1
Enter the name of the molecule or the chemical formula for the molecule in the block against the title “Molecule” for which the Lewis structure is required.
The molecule contains the atoms of the same element whereas a compound contains atoms of various elements. This calculator can take in both the molecules and compounds and output the Lewis structure for it.
If the user enters an ionic compound such as NaCl, the calculator prompts, “Lewis structures do not apply to molecules with ionic bonds” in the results window. Ionic compounds contain positive and negative ions with ionic bonds between the atoms. They do not deal with the sharing of electrons.
Ionic bonds are formed between atoms by giving or taking a valence electron. This calculator does not support the ionic bonds and only deals with covalent bonding which refers to the Lewis Structure.
If the user enters a molecule with the wrong spelling or the chemical formula for the molecule is not correct, the calculator gives the signal of “Not a valid input, please try again.” The user can easily identify the incorrectness in the input by this signal.
The Lewis Structure Calculator sets H as the default input for the calculator. This is the chemical formula for the hydrogen atom. Though a single atom does not have a covalent bond, the calculator shows the structure of the atom by showing its valence electrons through the dot structure.
Step 2
After entering the molecule in the input tab, the user should press “Submit” for the calculator to process the input molecule. The calculator loads the result and shows “computing” in the output window.
It takes a few seconds for the calculator to output the result in a new window.
Output
The Output window of the Lewis Structure Calculator shows the following tabs in the result window:
Input Interpretation
The calculator makes the input interpretation from the molecule entered by the user. The input interpretation shows the name of the molecule which the user entered in the input tab.
It can also show the chemical formula for the molecule which the calculator assumed from the input. With the name of the molecule, the calculator also displays “Lewis Structure” in this window.
Result
The calculator displays the Lewis dot structure of the entered molecule in the Result window. The structure of the molecule is formed by first selecting the central atom. The atom with the lowest electronegativity is in the least number and has the largest size is the central atom of the molecule. The calculator selects the central atom by viewing the above factors of all the atoms in the molecule.
Hydrogen and fluorine can never be the central atom. The central atom can have eight or more electrons. The central atom shares the maximum number of electrons. The central atom tries to remain in maximum covalency.
The octet of the corner atoms must be complete for the Lewis structure. If the molecule contains a positive charge, it will always be on the central atom. For example, in the case of hydronium cation $H_{3} O^{+}$, the positive charge will be on oxygen O.
If the molecule contains a negative charge, it will always be on a corner atom. For example, in the case of sulfate anion ${SO_{4}}^{-2}$, the two negative charges will be on the two corner oxygen O atoms.
The calculator displays the results according to the rules given above.
Solved Examples
Here are some examples of the Lewis Structure Calculator.
Example 1
For the ammonium cation $N{H_{4}}^{+}$, draw the Lewis structure showing all the valence electrons of the atoms.
Solution
The user enters ammonium or the formula for ammonium cation $N{H_{4}}^{+}$ in the input tab of the calculator. The calculator processes the input and displays the input interpretation.
The input interpretation consists of the name of the ammonium cation and the structure required for the cation. It shows the “Lewis Dot Structure” written alongside the ammonium cation.
The Result window of the calculator shows the Lewis structure for $N{H_{4}}^{+}$ as follows:
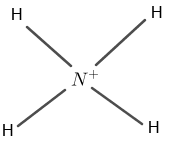
Figure 1
Nitrogen has five valence electrons and hydrogen has one valence electron. The molecule also has a positive charge on N in $N{H_{4}}^{+}$.
The central atom is nitrogen which is bounded by four hydrogen atoms. Four hydrogen atoms surround the nitrogen atom by four single covalent bonds.
Through the single covalent bonds, nitrogen has complete eight electrons in the valence shell and hydrogen has complete two outer-most electrons as shown in figure 1.
The positive sign on nitrogen indicates an extra electron on nitrogen.
Example 2
The nitrate anion has the chemical formula $N {O_{3}}^{-1}$. Draw the Lewis structure for the nitrate anion showing all the valence electrons of all atoms.
Solution
Nitrate anion or $N {O_{3}}^{-1}$ is entered into the molecule input tab of the Lewis Structure Calculator.
The calculator gives the input interpretation of nitrate anion if the user enters the chemical formula $N {O_{3}}^{-1}$ in the input tab.
The input interpretation also displays the “Lewis Dot Structure” written alongside the nitrate anion.
In the next window, the Result is displayed showing the Lewis dot structure for the nitrate anion $N {O_{3}}^{-1}$ as shown in figure 2:
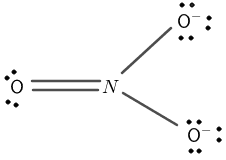
Figure 2
Nitrogen has a total of seven electrons and five electrons are present in its valence shell. Oxygen has a total of eight electrons having six electrons in its valence shell.
The central atom is nitrogen and it is bonded with three oxygen atoms. The three oxygen atoms surround the nitrogen atom through covalent bonds.
The dots on the oxygen atom are remaining valence electrons that do not take part in covalent bonding.
The oxygen completes its octet by forming a single covalent bond sharing one electron with the nitrogen atom.
The nitrogen octet now consists of six electrons, its octet is not complete. It makes a double covalent bond with one of the oxygen atoms to complete its octet.
The negative charge is placed on the two corner atoms of oxygen making single bonds with the nitrogen atom. The complete structure is shown in figure 2.
Therefore, the Lewis Structure calculator helps to draw the Lewis structures of different molecules easily.
All the images are created using GeoGebra.
